Formlabs BioMed Flex 80A Resin
- Express Dispatch
- Australian Owned & Operated
- Money Back Guarantee
- Quality Assured Products
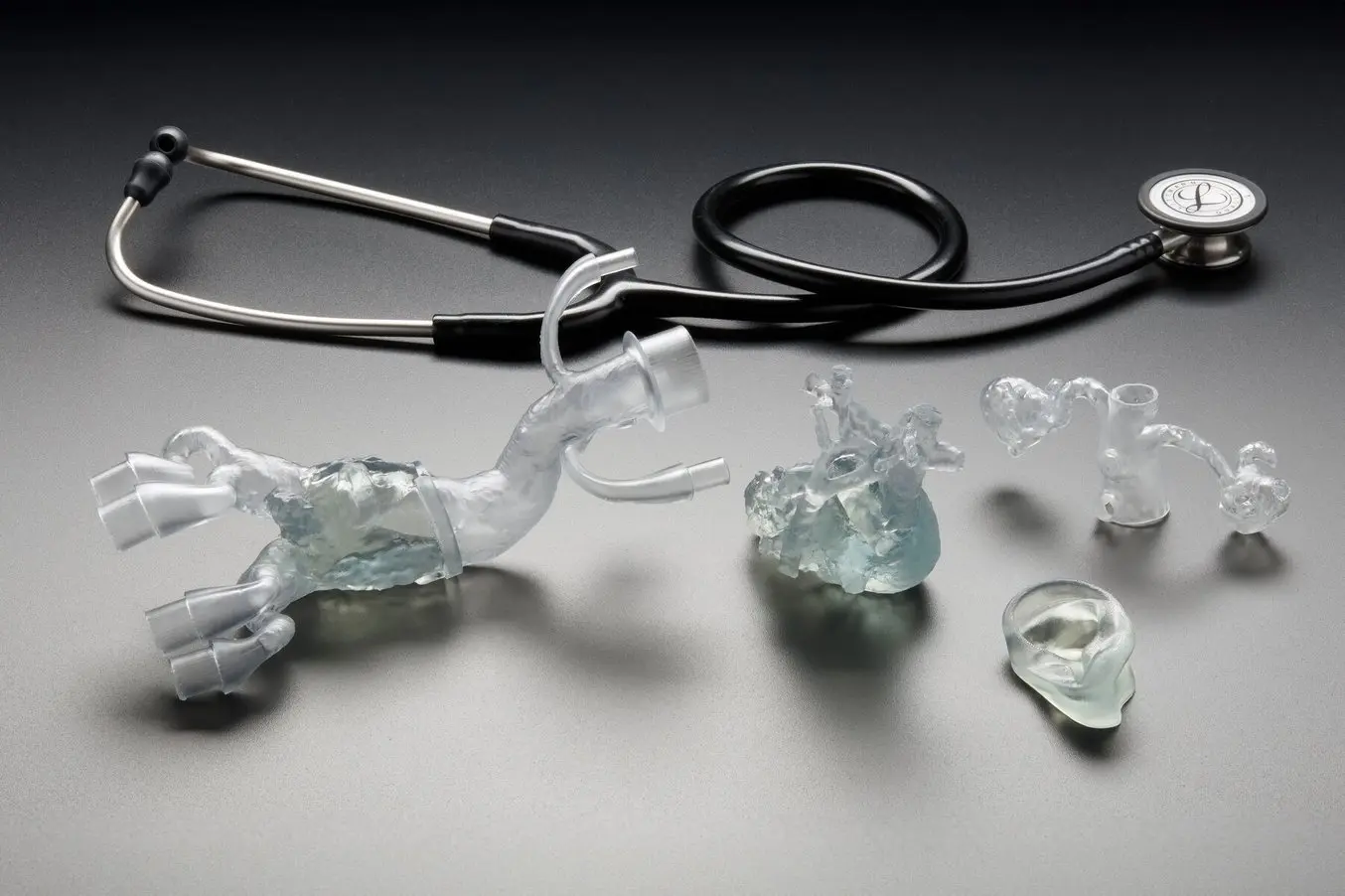
Formlabs BioMed Flex 80A Resin
A firm, flexible, transparent biocompatible resin for durable parts — designed for long-term skin contact (>30 days) or short-term mucosal membrane contact (<24 hours).
Why choose BioMed Flex 80A Resin?
Firm flexibility for durability
A tougher, more supportive flexible material for parts that must hold up over time.
Transparent for inspection
Clear parts help with visual checks while still providing flexible performance.
Biocompatible indications
Supports long-term skin contact (>30 days) and short-term mucosal membrane contact (<24 hours) when processed per validated workflows.
Medical-grade manufacturing
Produced in an FDA-registered, ISO 13485 certified facility for improved traceability and confidence.
Applications
Use BioMed Flex 80A Resin for durable flexible parts where firmness, transparency, and biocompatible indications matter.
- Durable patient-contact components (long-term skin contact >30 days)
- Firm flexible interfaces, clips, and compliant features
- Transparent flexible components for visual alignment/inspection
- Short-term mucosal membrane contact parts (<24 hours) when processed per validated workflows
Note: Indications and performance depend on validated printing + post-processing. Always follow the IFU and validate your process for your specific application.
Workflow overview
Standard SLA sequence of wash → dry → post-cure, with strict discipline for biocompatible use: dedicated validated equipment, following the Manufacturing Guide/IFU, and internal validation for your application.
Typical validated post-processing summary
- Wash: Use the validated wash method/time in the IFU for clean, consistent surfaces.
- Dry: Ensure parts are fully dry before curing.
- Post-cure: Follow the validated cure cycle in the Manufacturing Guide/IFU for intended performance and indications.
Best practice: Always follow the official Manufacturing Guide/IFU for your exact equipment and workflow.
Sterilization & disinfection
Refer to the official documentation for any validated compatibility guidance with disinfection and sterilization methods.
Important: Material properties vary with geometry, settings, and sterilization method. Validate your full process for each application.
Tech specs at a glance
Tip: Use the official Manufacturing Guide/IFU for validated settings, post-processing, and any sterilization compatibility guidance.
Downloads & documentation
(70778)
| SKU | 70778 |
| Brand | Formlabs |
Be The First To Review This Product!
Help other 3DPrintergear users shop smarter by writing reviews for products you have purchased.